Foreword
“In the beginner’s mind there are many possibilities, but in the expert’s there are few.” – Shunryu Suzuki.
Sometimes, something is so ordinary, ubiquitous, and seemingly dull – or scientifically passé – that we fail to see the mysterious beauty before us. Even though we rely on this perplexing substance for our existence, we – the average public person – do not search for answers. We don’t even know there any questions to ask. Sometimes, as Suzuki tells us, it takes the ability to look at the world from a beginner’s mind to realize that even experts are lacking. Sometimes, we miss the mysteries or magic simply because we’ve always been surrounded by it.
Welcome to episode 8 of Remedial Polymath: Water: You Don’t Know Shit About It

A Story
Tanzania, 1963. The yoke of British colonialism had recently been lifted. America was in the midst of figuring out how to get men on the moon using machines containing far less computing power than many modern watches. The Beatles were about to change popular music forever. Unthought-of weapons such as the Hydrogen fusion bomb (an atom that is pertinent for our discussion), colloquially known as the H-bomb, hung over the heads of almost everyone. We eagerly looked to realize so much novel information about our possibilities, our world, our solar system, and beyond. And it was happening; we were excelling in a manner unknown to humankind before that time.
And just as how at times one can’t see the forest for the trees, at times we can’t see the trees for the forest, or maybe not even the soil beneath both. Because when it came to the substance that envelopes most of our planet, which we consume and interact with daily, we apparently had/have so much to learn. We didn’t know we didn’t know things, not to this level, about the substance we call water. That was in 1963, and guess what? We still have a dearth of knowledge about this necessary molecule that makes up 60% of our bodies.
60% of you is water. That is mind-bending if you stop and think about it.
Back to Tanzania. A 13-year-old boy named Erasto Mpemba was in his secondary school making ice cream in a class. Each student in the class was mixing boiled milk and sugar. Usually, you wait for the mixture to cool before you refrigerate it. Mpemba got bored and decided to refrigerate his mixture right away. Soon, he checked his ice cream and was amazed that the time it took his bag to become ice cream was quicker than any of his classmates who had waited for theirs to cool.
When he presented this surprising phenomenon to his teacher, he was disregarded, laughed at, and almost shushed away. He was told this observation couldn’t be possible. But Mpemba knew what he witnessed and wasn’t satisfied with this dismissal. He may have been bolstered by his findings from local street ice cream vendors who were more familiar with this phenomenon. Mpemba held on to this memory and his assertion.
Six years later, a physics professor visited his school. His name was Dr. Denis Osborne. Mpemba asked him bluntly during a Q&A why hot water freezes faster than cold. Again, he was chuckled at. Osborne was taken aback, but upon hearing this question, he decided to give the boy his attention. He probably also had to know if this could be correct.
They ran experiments, freezing beakers of water at different temperatures simultaneously. To the doctor’s surprise, Mpemba was correct that the hot water in their experiment froze quicker than the cold water. It took until 1969 for someone to formally notice this and present it scientifically. Their findings were then presented in an article in the Physics Education journal, where they were both listed as authors.
Like how gravity pulls on objects of different masses at the same speed, these observations don’t compute with our instincts. Even experts often don’t think of overriding those instincts. We collectively never even look into questions like this until someone throws some objects from a leaning tower in Pisa and does a straightforward experiment (that was Galileo, by the way).
We now call this water temperature phenomenon the Mpemba effect. And we know that it indeed happens. But astonishingly, until this day, we have yet to understand precisely why. There are many theories and even detractors, but no explanation has reached a consensus. That is what is fascinating, not so much the scientific facts as that such a simple truth about a “simple substance” is still hotly debated.
Perhaps even more astonishing is that Aristotle and Rene Descartes made vague references to this effect (without explanation, of course). So even with two men, two incredibly respected scientists/philosophers in our history, making references to this potential phenomenon, no future scientist decided to run an experiment. Humans just could not overcome what they thought was an obvious occurrence.
That is until a curious Tanzanian high schooler, unburdened with the domestication of science, decided that he wanted his ice cream faster. It took the mind of a beginner to challenge the experts. Because of that, we scientifically established something previously unknown about the most common liquid on Earth.
Now, it should be stated that the Mpemba effect is not universally accepted and lacks a definitive scientific explanation. It does not always occur in highly controlled scientific environments, such as having the containers in a vacuum where evaporation wouldn’t happen. But we don’t live in a vacuum, and in many everyday situations, it certainly does occur. You may have seen videos of people throwing water into the air when it is extremely cold out, and it freezes before it hits the ground; that is done with hot water, not cold. I have read that “further research is needed.” That’s the fantastic part; it’s 2024, and further research is needed to understand water freezing.
Nevertheless, it’s a stellar and fitting story.
So, let’s be like Erasto Mpemba, be curious, and examine this beautiful, life-giving molecule. There is much we need to learn, and even more in which we can scientifically tell the ‘what’ but not the ‘how’ or ‘why.’ Either way, I’ll wager you have never been exposed to this information.
I certainly was not.
When you realize you’re surrounded by magic and mystery in your everyday life, that’s a fun topic worth exploring. And what’s more every day than water?
Please help
If you are still listening/reading this episode I would think you at least don’t hate it.
The purpose of this show is to give you all a bit of fun and wonder while we explore a variety of topics. I aim to challenge you while keeping it accessible. I do this for everyone by doing a lot of researching, reading, writing and re-writing. There is currently no money in it.
So I ask in return for you to take 10 seconds out of your day and rate and review it on any podcast app you use but especially if you use Apple Podcasts. Even if your review is ‘Good Show’. If any episode or topic is to your liking, please share it on any social media app or website you fancy. I need your help in this regard!
But even more importantly, could you tell those in your life about the show if you are enjoying it. Nothing beats word of mouth.
You listeners who have found the show while it is just a baby, I love y’all. Any all all assistance is deeply appreciate.
Okay, back to discussing water. Let’s dive in and make our way to the deep end of this pool.

Introduction:
Despite our utter dependence on it, water is a puzzle. Scientists don’t understand precisely why it expands as it cools, why hot water can turn to ice faster than cold water, or its extraordinary surface tension, which allows it to cling to soil and support insects. “It’s almost like water is trying to hide its secrets,” says John Russo, a mathematician at the University of Bristol in England, who thinks he may have figured one out.
“The structure of water – the reason for its peculiar properties – is a major question in chemistry and physics,” said Richard Saykally from the University of California, Berkeley.
But let’s back up a bit.
‘Water’ has slightly different meanings in different languages and cultures. In some, the word unsurprisingly also means ‘life’; in others, it also means ‘flow’; in others, it is the same word as ‘rain’ or ‘river.’ Its importance and secrets to our ancestors mean its definitions sometimes jump meanings. For this discussion, we will steer toward discussing water as the H20 molecules instead of large water-related systems.
That being said, my favorite different meaning for water is ‘flow.’ That was in Sumerian, where this word was also used for the number 1 and the letter ‘a’ (although that wasn’t the “first” letter as we think about the alphabet). Still, that is bewitching information from one of the oldest civilizations we know of.
Because of Earth’s average temperature, water’s liquid form is the most common, although we know it can easily transition into a gas or solid through temperature changes. The clouds we see in the sky are just water in a different form. The ice in your fridge is also (I know, that’s no surprise, stay with me). Those of us in developed countries often experience it in all its forms daily without thought. We know need it to live, sure, but we think about it little more than that.
I have yet to tell you anything you don’t already know.
Did you know that of the naturally occurring liquids on our globe, it effortlessly makes the world’s winners podium for the strangest? It acts in many ways unlike anything else. Plus, this isn’t just curious; if water wasn’t so bizarre, we wouldn’t be here. Human life, and most Earthly life in general, wouldn’t be possible.
The substance life requires to fend off death can only support life due to it being so anomalous. Do you believe I am now being ambitious and hyperbolic for the sake of podcasting?
This episode will just scratch the surface. We’ll keep this “simple”; it could have been much longer, but I wanted to mix things up from the last couple of topics.
Consider this: When compared to most other liquids, scientists tell us water has 66 properties that the vast majority of other liquids do not possess. We will not explore all of water’s 66 wild properties, don’t worry. But let’s take a look at some of the prominent ones.

Where has it come from?
One should begin at the beginning, of course.
Where does our water come from?
For Earth, it is improbable that, like most else on our planet, the entirety of our water is from the initial planetary formation. Not entirely, at least. When our planet was more akin to a ball of lava a bit over 4.5 billion years ago, it was a sweltering place without an atmosphere. If any water was within that fiery ball, it may have been vaporized and volcanically ejected, which would have led to it being blown into space by the solar wind. We could have had the precursors for water (hydrogen and oxygen), of course, but again, the water would have become a gas and been blown away from the hot protoplanet without an atmosphere. Once the planet cooled, there could have been some beneath the Earth’s crust that found its way out, but that doesn’t explain the vast amount we witness today.
We are left with a water delivery system, which is still out there but was much more common at this stage of our solar system; comets and asteroids. To cut to the chase, from testing the ratio of “heavy” hydrogen (that with a proton) within asteroids to the water on Earth, we see that they match up well. Much better than the water from comets (even though we can’t 100% rule them out also). So asteroid impacts, post Earth cooling, best explains why so much water is here. It may also help explain why Venus and Mars do not show as much water. They were not treated to the same collection of soggy asteroids.
You may have heard that you are made of stardust, and that’s true; everything in our solar system comes from the explosion of what existed before our sun. But Earth’s water – or water ingredients – seems to come from an asteroid or two visiting our planet after it cooled down. So, while most atoms in your body may be from a solar explosion, it seems almost every molecule of water – 60% of your body – comes from asteroid travelers to our planet billions of years ago. This is interesting because I knew Earth was the right size, with the right ingredients, at the perfect distance from the ideal type of sun; it wouldn’t have been enough. We also had to be hit with the right asteroids at the right time.
To say our home planet is lucky or unique is to do a disservice to those words, as we’re light-years beyond the meaning of those words when describing the anomalous planet we come from.
Was it only from asteroids? Probably not. Would we have life on Earth without asteroids? Probably not.
Water’s Weird Ways that We Can Kind of Explain (But Are Still Wild)
What is water?
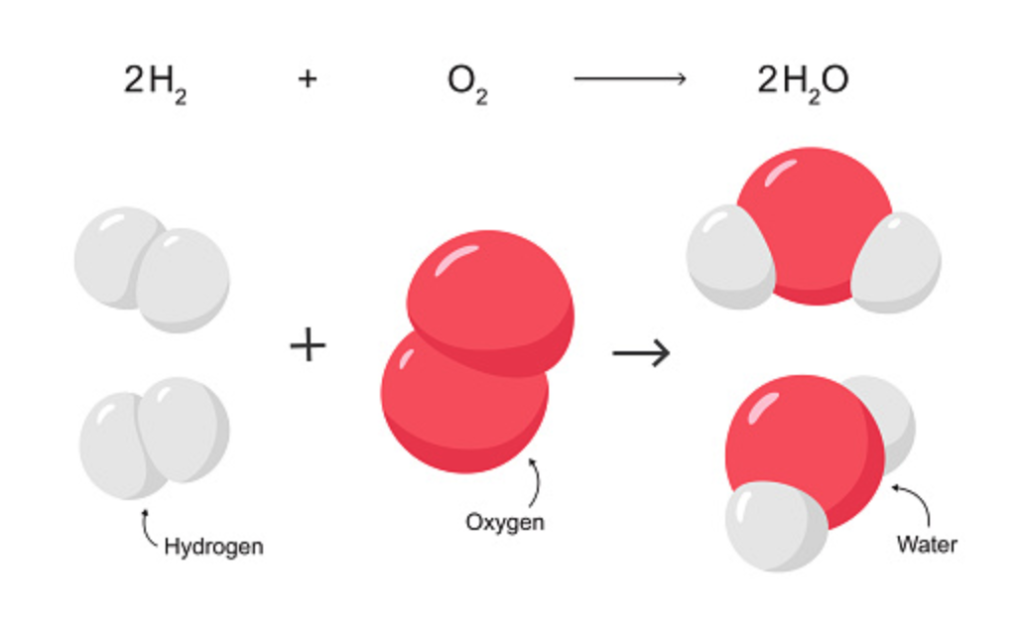
Let us now discuss something a little closer to Earth. Let’s examine first simply what water is. I don’t have to tell you that water is two Hydrogen atoms and a single Oxygen atom. Oxygen has eight electrons, the negatively charged particles that orbit the center of the atom, and hydrogen has one. When they meet, they create a letter V of sorts, with oxygen at the bottom of the V and the hydrogens at the top. The hydrogen atoms grab an electron from the oxygen atom, and this holds them in place as a molecule. This is called covalent bonding. We will come back to that later.
Sidebar: Is it not wild that if you separate water’s two components, Hydrogen and Oxygen, you are left with gases that, when combined with a spark, will lead to massive explosions. But of course, when combined into water, you have a molecule that can put that flame out. I just find that interesting.
In H2O, the hydrogen atoms have a very strong covalent bond with the oxygen atom. Through the sharing of electrons, they both fill each other’s ‘electron shells,’ which we don’t have to go into; just know it’s a powerful bond. These hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other slightly negative ions. Again, it’s not essential to understand that. Just know that, in short, water molecules are also attracted to each other. This hydrogen-to-hydrogen attraction has some interesting and unusual side effects.
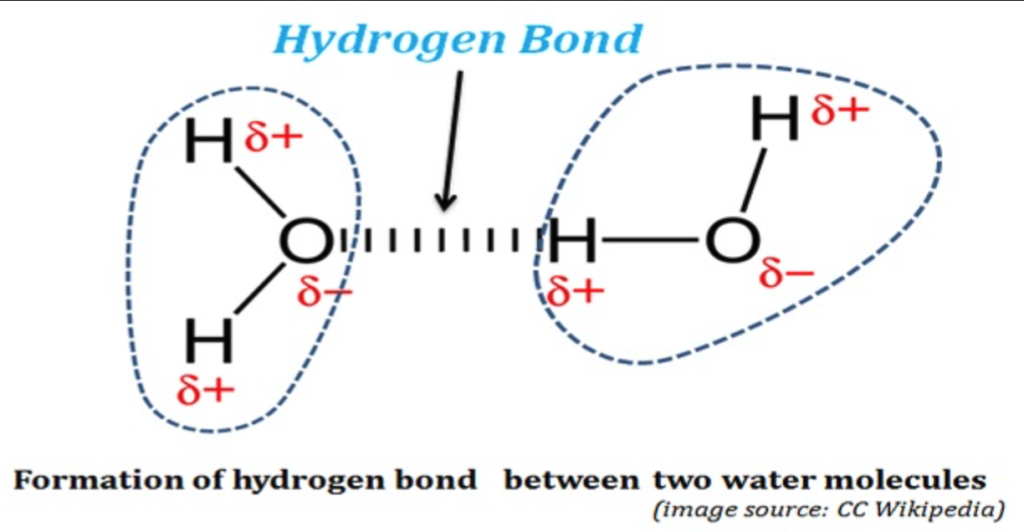
Sidebar: It is actually the strength and regularity of the hydrogen bonds that keep water from being a gas at regular atmospheric pressure and typical ambient temperatures. In contrast, hydrogen sulfide (H₂S), ammonia (NH₃), and hydrogen chloride (HCl) are three light molecules, like water, but at room temperature, despite being similar to or greater than water in weight, do not have hydrogen bonds as effectively as water does and hence are gases.
Stay with me.
Now, let’s examine the shape water molecules like to take when put together in their liquid state.
The hydrogen atoms in a water molecule can be hydrogen bonded with up to four other water molecules. I have heard this described as 4 Vs attached to each other, basically a 3D pyramidal shape (with one molecule in the middle of the pyramid). The formal name is a tetrahedral. This is not a common occurrence, and while we understand how it’s done, we don’t know why, as other liquids do not behave like this, even with similar properties.
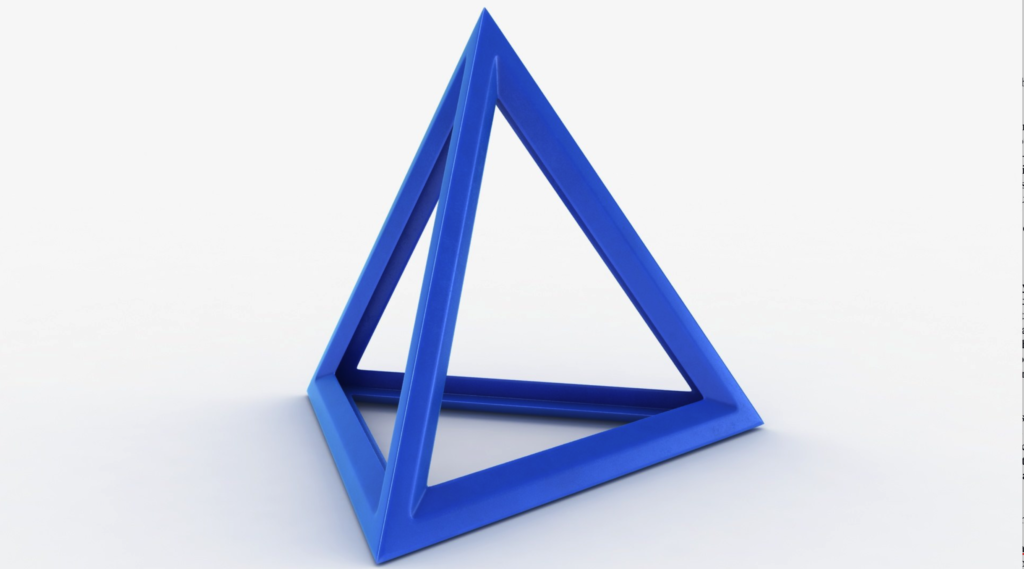
Sidebar: It’s fascinating that the molecules of the liquid water we need to survive take the shape of a pyramid. It forms itself into a shape that has always fascinated mankind. The shape of some of our oldest and most mysterious structures found on every continent. Did the ancients know something, somehow, that we have forgotten?! I am never one to put such symbolic mystery past our ancestors. Either way, what a fun “coincidence.”
There are other polar liquids with hydrogen bonds (e.g., ammonia, ethanol, hexane). However, they do not form the extensive & highly organized tetrahedral network seen in water. There is an interesting addition: the hydrogen bonds between H2O molecules are clearly weaker than covalent bonds within H2O. They are about 10 times weaker. This means that the attachments between the hydrogen atoms can form, break, and reform quickly in liquid water. But much of the way they break and form hold s many of the mysteries of this substance. More on this later.
The heaviness of water??
What does this mean? So, while 4 liquid water molecules like to join together into this 4-sided 3D pyramidal structure, there can also be others with 3, 2, or no bonds with other water molecules. The number of hydrogen bonds formed per molecule varies, and that can vary depending on what the water is doing. This ability to create complex hydrogen bonds between the water molecules without guaranteeing complex hydrogen bonds is not common.
So what do these odd rules that H2O plays by mean for us and our world?

Water is Sticky
Formally, we say that it has a high amount of surface tension. Actually, it has a ridiculous amount of surface tension. We’ve all witnessed this but may not have given it much thought. This is why bugs can walk about on the surface of water, and even a paper clip will “float” on water if it is correctly placed.
Okay, bugs and paper clips aside, what does this mean for us? Good question.
This surface tension allows water molecules to flow through the smallest blood vessels in the human body (even when fighting against gravity), grabbing oxygen, nutrients, and the like along with them. It allows for plants to suck up water from deep down within the ground. It allows the soil to easily retain water, making it available for any type of plant for a relatively long time. Those are attributes for which we should all be thankful for.
Stickiness also plays into, but isn’t solely responsible for, water’s incredible weight. Water’s density is relatively off the charts. The same hydrogen bonds that help make water so sticky also lead to it being so dense and weighty. Think about it, a gallon of water weighs a whopping 8 1/2 pounds (or around 3.8 kilograms). This also leads us to another interesting and unique fact, water is incompressible. It’s volume doesn’t change under pressure, even immense pressure. Whether you are at the surface or visiting the Titanic, the weight vs volume stats for water are essentially unchanged. Not only is that fascinating it also means that water retains it’s density across any conditions.
Just think about how useful this fact has been for us humans. It means we can load up a ridiculous amount of heavy material, or a serious amount of people, onto relatively little boats and move them easily to where they need to get. Just think of a US air craft carrier, they weight around 100,000 tons, and yet they float not only easily but with plenty of height to spare, usually being having a deck above water equal to a 20 story building.
This weight of water also helps us with hydraulic power, water distribution systems (think those water towers you have seen while you drive on the interstate), the fact that water sinks down to aquifers and ground water for wells for people and farms, or the fact that it it assists with erosion and soil fertility (which is a process that fertilizers our farms and shapes landscapes, forms valleys, deltas, and other landforms that are beneficial for human settlements).

Universal Solvent
Another interesting attribute of water that is almost as important is that it is an excellent solvent due to its hydrogen bonds and interesting shape in liquid form. It doesn’t just stick to itself well but to almost anything else as well. This means there is little competition when it comes to tearing other compounds apart. It’s virtually a universal solvent. Nearly every compound we know of will dissolve in water at least slightly, which is wild if you stop to think about it. It’s probably the best solvent we have on Earth. So much so that it becomes challenging to create absolutely pure water, even in a lab setting. Think about that!
This ability is crucial for life. Nutrients and oxygen can reach where they are needed in our bodies, and poisons that need to be ejected from our bodies can all move to where they need to go because water can dissolve and easily transport them. Our DNA and the membranes of the cells within our bodies won’t work without water. And it gets cooler than just its ability to dissolve.

DNA and its double helix couldn’t exist without water, and its surface is always covered by water molecules. But I think it would be better to quote from an article from Science Daily on water and DNA: “The DNA’s double helix never occurs in isolation; instead, its entire surface is always covered by water molecules which attach themselves with the help of hydrogen bonds. But the DNA does not bind all molecules the same way. ‘We’ve been able to verify that some of the water is bound stronger whereas other molecules are less so,’ notes Dr. Karim Fahmy, Head of the Biophysics Division at the Institute of Radiochemistry. This is, however, only true if the water content is low. When the water sheath swells, these differences are adjusted and all hydrogen bonds become equally strong. This, in turn, changes the geometry of the DNA strand: The backbone of the double helix, which consists of sugar and phosphate groups, bends slightly. ‘The precise DNA structure depends on the specific amount of water surrounding the molecule,’ summarizes Dr. Fahmy.”
This means our DNA’s exact shape depends on how much water is present. Not only do your organs and body need water, but your DNA’s geometry will change without it. I know this may be “eye-rolly,” but I didn’t just say we need water. We are so intertwined with it that without it, in a short time, the geometry of our DNA changes, and the DNA seems to start making decisions about which part of itself needs water more than others. That is straight trippy.
I’ve never heard a better argument for hydration than that.
Water and Heat
Another interesting side effect of the tetrahedral shape of liquid H2O and the bonds within it is water’s fantastic ability to hold onto heat. This, in turn, is why water is an excellent coolant and why it has been used to cool down everything from nuclear reactors to planes and cars to servers and data centers. So, while it can absorb a lot of heat, it also takes a serious amount of heat to actually change the water’s temperature. And then, in turn, it can take a long time for that water to cool down.
When water is exposed to heat, that extra energy goes into converting more and more molecules from its tetrahedral structure to other more disordered structures. So the heat doesn’t go immediately into kinetic energy (i.e., temperature) but into a change of the water molecules’ shapes, at least at first. This is not a common reaction for a liquid. For this reason, water also has a very high boiling point compared to other liquids.
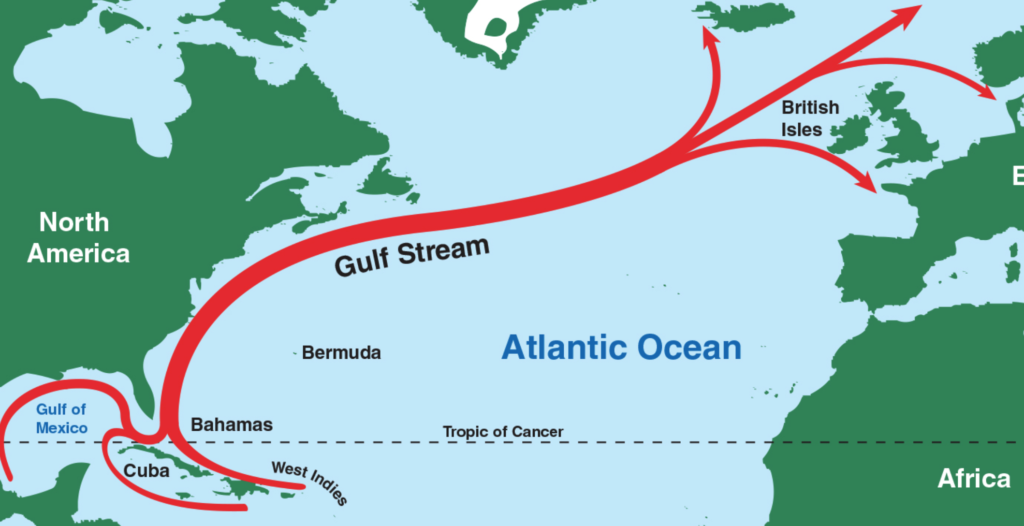
This plays a massive part in explaining why Earth is the way it is. It means the oceans take a long time to heat up and cool down. This, in turn, means that they play a significant role in being a moderator of sorts for our climate. This is why, on balance, areas near the oceans rarely get very hot or very cold. It is why the Gulf Stream works and why Northern Europe (latitude-wise, almost the whole of Europe) is currently as warm as it is. This is why, despite Chicago and Barcelona having a similar latitude, they couldn’t have more different climates. The Caribbean is a long way away from England, yet the Gulf Stream ocean current water stays, warm enough to have a massive impact on the weather there. Oceanic currents such as this around the world are vital for moving heat and regulating temperatures.
This manner in which water absorbs heat also leads to stable evaporation instead of water solely increasing its temperature through increased kinetic energy like other liquids would do. This means that the water in the oceans can deconstruct the molecular forms within it – without ever getting anywhere near boiling – and then release those non-connected water molecules into the air without the water barely changing in temperature. This phenomenon is again due to the unique pyramidal shape in which the H2O molecules like to arrange themselves. This unique aspect of water means we have abundant clouds, humidity, and precipitation, even if you’re far from a body of water. Of course, these weather effects make it possible for the weather to be regulated almost everywhere and for freshwater to fall from the sky as rain, which practically every terrestrial living creature relies on. All in all, the ability of water to retain heat and not change too quickly is why we have a (for the most part) stable and diverse ecosystem all around the world.
Sidebar: This is not me saying that we have a stable and diverse ecosystem everywhere or that global warming isn’t a thing. With the massive temperature changes via the sun the Earth experiences, looking at everything relatively, no other liquid would keep things as stable as they are here on Earth. Most humans, wherever they are, collectively live within a temperate range that is only about 50 degrees Celsius. That isn’t a large number, and it is all thanks to water’s properties.
Liquid Water and Ice
Now it is time to discuss one of the most significant mysteries surrounding water, which has massive implications for life on Earth, and it is my favorite. It is the trait of water that has always fascinated me and inspired this episode.
Water, when it freezes, expands. There are very few liquids of any kind that expand upon freezing. Water is the only commonplace liquid that does so. While we generally understand the mechanism of this phenomenon, we do not have a good idea of the ‘why’ behind it. Thank God water is odd in this way, though. Otherwise, I wouldn’t be talking to you right now, and you wouldn’t be listening/reading.
Anders Nilsson is a chemical physicist who works at Stockholm University in Sweden and Stanford University in the US. He says, “If you look at the simple thermodynamic and kinetic properties of liquids, as you change pressure and temperature, they all behave the same way.” You see, usually, as you cool off a liquid, its density will increase, and its heat capacity and compressibility will decrease as you near its freezing point. Anders then tells us, “Almost all liquids on the planet behave like that. Except for water.”
But here is an almost funnier aspect of the story: Water does kind of do what other liquids do. As you lower the temperature of, let’s say, room-temperature water, its density will increase just like other liquids. This will happen until you hit 4 degrees Celsius, and then bam, just like that, water reverses course and decreases in density. Why 4 degrees? Why reverse course? It’s yet to be 100% known why H2O plays this trick on us.
And then, when it hits 0 degrees Celsius and freezes, it will be decidedly less dense than liquid water of any temperature. What does this mean? Well, numerous things. Primarily, though, this means that frozen water weighs less than liquid water. It’ll float. As to the theories behind why, we will get to that eventually, but first, let’s consider the implications behind this phenomenon.
Sidebar: Now, I must relate another terrifying odd aspect of water freezing. Not so much as to illuminate the implications of water expanding its way ice upon our lives, but just to weird you out and again showcase just how peculiar the liquid-of-life can be. If you don’t feel that God, providence, or the universe (whatever you want to call it) is essentially winking at us with this substance, consider this…
We all know that water freezes at 0 degrees Celsius; that is why that is the zero point. However, technically, that is water with any impurities, i.e. all natural water on Earth. However, that is not the case if you were to get yourself truly pure water. Should you find yourself with truly distilled or purified water, like that from reverse osmosis, then the whole zero-degree thing falls by the wayside. This is when you are dealing with “supercooled water.”
The arrangement of the molecules of liquid water determines its state of matter. We can say that the water is liquid when the H2O molecules are not touching and flowing freely. The water only becomes ice when the molecules come together and lock into place.
But it needs help. It requires some impetus to “lock into” place and freeze. It needs something to crystallize with. It needs a “seed crystal.” Without a seed crystal, the water has nothing to form a crystal around. It is as if liquid water doesn’t know how to crystallize without some impurity or movement, so it stays as is. Any kind of impurity or even movement of the water could set this off. There is never water with no impurities in nature, so this isn’t an issue.
I know. It’s bizarre.
Of course, with science, we can get pure water and lower the unmoving pure water well to below zero degrees. This water can go down to -48 degrees Celsius before freezing! That is a perfect experiment, as the exact freezing temperature depends on the shape of the container and its purity, but it often gets to – 20 degrees before freezing. Simply disturbing the molecules by shaking, pouring, or banging on a surface can catalyze the crystallization process as well as introducing any impurity.
I remember a chemistry experiment we did in high school. We had supercooled water and dropped an extremely small stone, almost a speck of sand, into the container, and—bam—it immediately all became ice. Within under a second…
Just bonkers.
We will discuss why this may happen for water when we discuss why water may expand upon freezing. For now, I wanted to weird out anyone unaware of supercooling water. And if you did, I wanted to remind you – and everyone – of how terrifically odd our life-giving molecule really is.
Gotta love it! Now, the sidebar is over, and we are back to our regularly scheduled content.
—–
Back to ice being less dense than water. One would venture to think most humans have never wondered at this occurrence. Ice floats in our drinks. One must remember not to put a water bottle in the freezer; it’ll explode. This aspect of water is just a known part of our lives. But, honestly, this is downright spooky. Consider other solids vs their own liquid form you have witnessed. Does solid wax float on melted wax? Have you ever witnessed solid butter floating on melted butter in a hot saucepan? What about fondue with cheese or chocolate? When you heat it up, do they float at the top of their liquid version?
Consider it is the ice age and ice sank instead of floated. It doesn’t have to be the ice age, but it’s more fun to go extreme to hammer home the point. Eventually, Earth would move out of the sweaty dinosaur times and into a colder period for one reason or another; we’ve plenty of proof that this would have to eventually occur.
Ice would be created on the surface and sink. This wouldn’t stop; layers of ice would slowly sink and accumulate on the seafloor. This would just continue and continue. There are many effects this would have on the planet. First and foremost, eventually, it seems that the oceans would become one massive ice rink outside of some equatorial regions. But who knows? Once you have a runaway ice formation, who is to say the heat at the equator would even be enough to counter the freezer-like effect of the rest of the world.
We could be relatively sure about this for a few reasons. There would be massive insulation loss. Normally, floating ice insulates the water beneath it. It moderates the water temperature beneath itself. This would be lost if the ice sank.
There would be a heat transfer issue. The cold air above would cool the water more efficiently without the insulating ice layer, helping to counteract any warming effect from the sun. This would probably create a feedback loop-like effect that would significantly impact our global climate. The heat exchange between air and water would be altered substantially. This would probably make much longer ice ages, or that would just become normal.
Clearly, we can’t know for sure. Just as clearly, this would have horrible implications for life. It is challenging to imagine human beings, not to mention any significant land-based animals, evolving to their current state. This cannot be stated outright, so I’ll state it quasi-outright. We won’t happen.
Let’s glance over some other predictions about the planet and life on it if water didn’t expand when frozen.
Aquatic life would have it bad. Not only would they lose the insulation that floating ice provides, but they would – in most places at least – just flat out be pushed out of their neighborhood. All the way from tiny plankton (listen to the episodes on seafood to see how massively important those little guys are) to massive whales. It may not be as big of a deal, but any type of life that requires floating ice, such as polar bears, seals, and penguins, would have a tough time existing and evolving at all.
Global freshwater systems or availability would have to be drastically different. The distribution and availability of freshwater would be entirely dissimilar to what we experience now. If the ice age glaciers never receded, we wouldn’t have many of the lakes and river systems we know of. If water is ice almost everywhere there is less liquid water to evaporate (which happens easier at warmer temperates) and less water becomes clouds and rain. There would be no, or drastically less, rainwater worldwide if the temperature never got warm anywhere. Who knows precisely how it would play out, but it is pretty safe to say that if ice sank and remained frozen on the ocean floor up, there would be a lot less freshwater, which – ya know – would affect the evolution of all terrestrial life. You’d have to imagine it wouldn’t positively impact the evolution of life in any way.
Listen, I like to think life finds a way because that is what we witness around us. However, it is just a massive stretch to think that with ice not expanding, we ever have a world in which – let’s say post, dinosaur death from an earth-cooling asteroid – small moles evolve into larger mammals that evolve into Savannah roaming bipedal humanoids that evolve into Homo sapiens.
As an article at reptileknowledge.com put it interestingly, “Thus ice floats because we are here to witness it floating.” This implies a philosophical idea similar to a conclusion I have come to after learning more about our life molecule; that is, the physical laws and properties we observe, such as ice floating, are inherently tied to our existence as observers. In other words, the universe must have the properties it does because otherwise, there wouldn’t be observers to notice and question these properties. Water, at least for life as we understand it, must have these odd properties for intelligent life to exist and bear witness to the odd properties.
I feel that water and its workings are another way the universe winks at us. It is the liquid that is the exception that proves the rule. This exception breaks the rules to ensure our universe is tilted towards creating intelligent life, at least on our orbiting rock.
—-
Sidebar: We could explore all day how water is unlike other liquids or how H2O is unlike other molecules. Remember, there are reportedly 66 ways water is scientifically unlike other liquids. We touched upon some of the big ones.
It is time to examine a theory behind why water may – may – possess some of the wild traits that it does. It is the potential ‘why’ that has been mentioned before in the episode. I love to explore this one, and it’s a good topic to end on. Not only may it be a reasonable explanation, but it also invites more philosophical thinking about life, human life, and the way the workings of the universe are embodied in water. I can’t help but explore examples of when science and philosophy have a crossroads.
Chaos and order
Let’s review some of what we discussed near the beginning of the episode. A water molecule can be compared to the letter V, with the oxygen atom at the bottom and the two hydrogen atoms at the top. The hydrogen atoms and the oxygen atom share some of their electrons, which are negatively charged, creating strong covalent bonds. The oxygen atom attracts the shared electrons more strongly, making that side of the molecule slightly negative and the hydrogen side somewhat positive. You have a minuscule magnet now, ready to connect with other molecules like itself.
Water seems to like to link up, especially as it cools, because each molecule is attracted to its four nearest neighbors using this magnet-like effect. The resulting shape is a three-sided pyramid or a pyramid where each side is a triangle, which is the shape we previously discussed, a tetrahedron (again, with one molecule at the center). Not only is this interesting, but it’s such a beautiful shape. It is also fascinating how it completely embraces the number 3, triangles, and pyramids, which have fascinated humans for time immemorial. I realize that may make many of you roll your eyes. Roll away. It is interesting, nonetheless.
But I digress…
While water’s ability to arrange itself into this shape is well understood, what happens after that is not totally agreed upon. In researching for this episode, it was wild how much educated people disagreed on the reasons behind water’s wild behavior. And to what level they disagree. It is utterly fascinating. It reminds me of parts of studying astrophysics, in which it seems that at a certain point, one’s personal philosophy plays a part in how one thinks the science plays out. When Anders Nilsson (the chemical physicist from Stanford) addresses this, he says, “It [the science of water] is bringing out very strong, almost religious opinions among different scientists.”
Let’s try to understand what the issue is. Water has hydrogen bonds, which are relatively strong and directional. As Martin Chaplin, a chemist and water researcher (yes, that is a thing) from London South Bank University in the UK, said, “It’s a lot of hydrogen bonding for such a small molecule.” And while water behaves relatively similar to many liquids when it’s warm, the biggest mystery is why it changes so much when it cools. This is when the hydrogen bonding starts to make a significant impact. Chaplin says hydrogen bonding “actually force[s] a structured, ordered state.”
Now, this is rare for the vast majority of liquids. Usually, the molecules are constantly rearranging in a liquid. Not water, and it’s because of the hydrogen bonds and its propensity for arranging into a tetrahedron. But it doesn’t have to be organized into a tetrahedron. That’s not a rule per se; as we touched upon earlier, you can have molecules of water that only attach to one other molecule, or two, or three, or none.
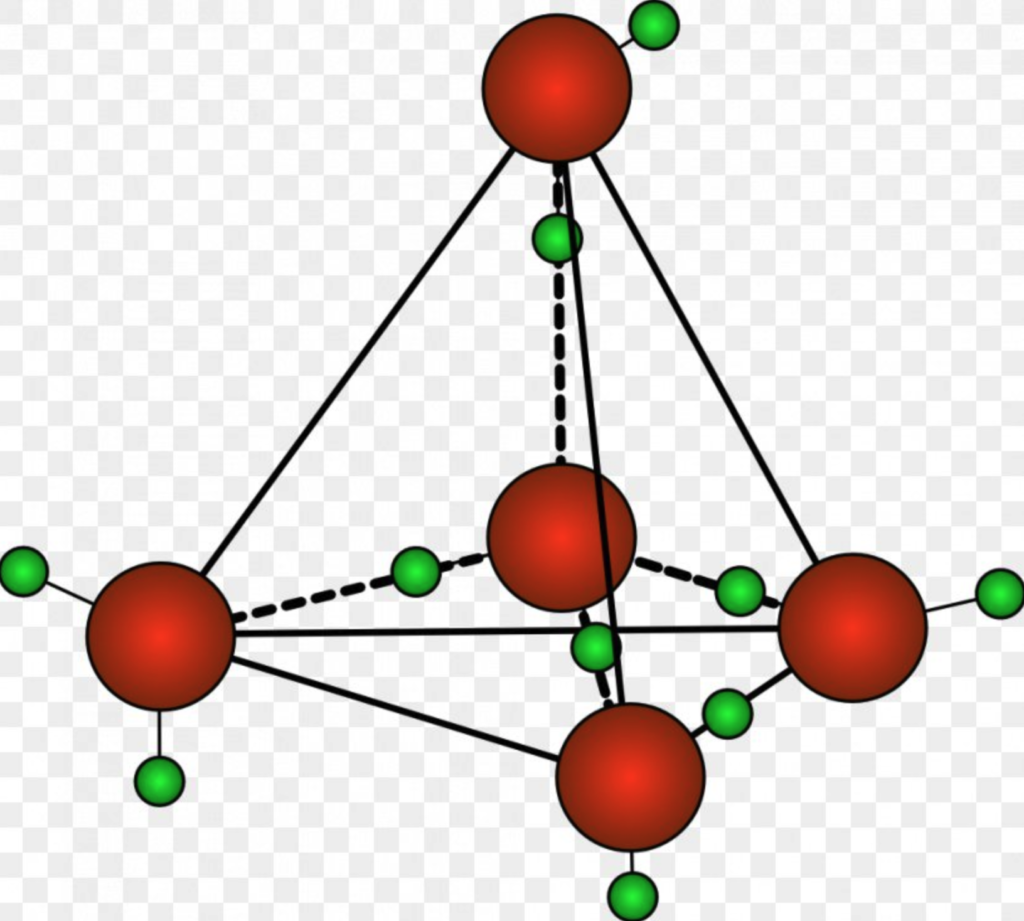
There can be many single molecules of water or even molecules that arrange into tetrahedrons, but so do in a less structured and interconnected manner. For this reason, many believe that water can, in essence, be considered two liquids in one, or to put it more correctly, the molecules in water can exist in two phases at once. The main difference between the two phases is thought to be the hydrogen bonds holding the molecules together. In one, four water molecules are arranged in an organized and symmetrical tetrahedron. In the other phase, the water molecules are disordered and interconnected differently, often incomplete. They also have different densities; the low-density version is the tetrahedron, which is less ordered and ice-like. The higher density one is with water molecules floating around in a non-structured way, which has a higher “packing” ability. Let’s not get too bogged down in the science here; the critical part to understand is that in this theory, the water molecules of water essentially arrange themselves in two ways, one is more orderly, and the other is more chaotic.
While this idea has gotten a new boost with modern tools and computer power, surprisingly, it isn’t new. There was a German physicist, Wilhelm Röntgen (actually, he was the one to discover x-rays), who, in 1892, proposed that water was made up of two distinct phases that coexisted in a mixture. He came to this conclusion using X-rays, and while his reasoning has been found incorrect, the theory has never left us.
In the 1990s, the two-phase model of liquid water got a new boost as computer power increased, allowing scientists to attempt to replicate the actual odd behavior of water. These computer models would change aspects of water molecules to see the effect. Importantly, they would change the strength of the hydrogen bonding that leads to the tetrahedral shape. John Russo, a mathematician at the University of Bristol in England, tells us that by doing this, “Many anomalies will essentially disappear – you can essentially make water behave like a simple liquid.” In one example in which they altered the strength and shapes of the hydrogen bonding, he tells us that ice indeed becomes denser than water and sinks instead of floating by increasing water’s ‘tetrahedrality’ (the proportion of molecules perfectly aligned with its four nearest neighbors). We know how important that is for life on Earth, so it seems that Russo is proposing that without the chaotic side of water, we probably wouldn’t be here.
Digest that interesting little idea.
In this hypothesis, the proportion of orderly tetrahedrons to more chaotic ones (or unorganized molecules) changes with temperature, with more low-density water forming as temperature decreases. This results in a sort of competition between the increase of density with decreasing temperature and the formation of low-density water. This could explain why water’s density maximum is 4 degrees Celsius, in addition to many of water’s anomalous properties.
But here is the wild thing, it’s still a hypothesis. Many scientists and researchers disagree with this idea. Many believe that water can only exist in one phase at a time, that this is just flat out not how liquids operate, even for one as weird as water. They think that the answers to water’s anomalies have yet to be found and that we may need better technology to find the answers. That blows me away. There is a good chance we may master quantum computers before we understand water, the most abundant and necessary liquid on Earth.
However, I like the chaos-and-order theory. Now, I am in no position to have an opinion on this matter. None. Yet I still do, and I’ll talk to you about it. And this is where the philosophy behind the science comes in for me. We’ve established that water has wild properties that are not understood, yet those wild properties make water life-giving for people specifically and life-giving for Earth in general. It makes sense to my mind that the reason beneath water’s beautiful properties is a mix of chaos and order. This would be so poetic and reflect how life seems to work, especially for humans. It strikes a deeper chord for me. It isn’t just having “fun” with the answers. It feels genuine, which, without better science, I would not be comfortable running with. It’s okay to go through life with thoughts such as this.
It seems odd to me that people would reject this theory with nothing to back it up except to say we have not yet found the key to unlock the mysteries. But mostly, it’s because of the answer’s poetic nature. It is that “wink” from the universe that intelligent life is just supposed to happen. It is Yang and Yang, light and dark, chaos and order. It makes sense in some wonderfully ineffable way.
A Little Fun
Speaking of Ying and Yang, it’s time to have some fun. I don’t usually want to needlessly jump into “woo-woo” on this show, so let’s say we are jumping into some “fun-fun.” While researching the tetrahedron, I was surprised to learn that Plato had also discussed it; it is considered one of the five ‘Platonic solids.’ He wrote about it in his work “Timaeus” almost 2,500 years ago. A Platonic solid is a shape in which all faces are identical regular polygons, the same number of faces meet at each vertex, and the shape is convex, meaning it has no indentations. There are only five of these shapes. The tetrahedron is unique in that it has the fewest faces, vertices, and edges. It is a shape studied for thousands of years for its mathematical beauty and symmetry. Interestingly, Plato associated it with fire.
Isn’t it cool that the shape water molecules like to form into has been considered beautiful and deeply thought about for a long time, well before humans knew about molecules? I think it is, and it is another thing that just kind of weirdly makes sense, in an amusing way, at least.
In numerous Eastern cultures, the tetrahedron is interpreted as a symbol representing the balance between the divine masculine and feminine energies, referred to as yang and ying in Taoist philosophy. When it points upward, it symbolizes the divine masculine or yang energy while representing action, direction, and initiation. When pointing downwards, it is associated with the divine feminine and symbolizes yin energy, receptivity, intuition, and nurturing. This duality is integral to many Eastern spiritual traditions, embodying the idea of harmony and balance in the universe. And, of course, chaos and order is also an essential duality.
In the world of sacred geometry, the tetrahedral represents a profound simplicity in the complex world of sacred geometry. It represents balance, transformation, and the pursuit of higher consciousness. It is said to be a reminder of principles that guide our path: change, stability, and the balance of opposing forces. That is also akin to chaos and order.
Now, I am not pushing those ideas on you. I just find it intriguing and entertaining. Not only is the shape water molecules like to take, one that has fascinated humans forever, but how water molecules arrange in their liquid state may poetically give it its anomalous properties. It’s fun food for thought for the vital molecule, just as when its atoms are separated, it can blow up but, when together, can extinguish.
Chaos and order, yin and yang, male and female, life and death.
Water can do both; nay, it has to do both to give life.
I don’t care what anyone says; it is just some recreational “science” worth spinning one’s wheels over at the end of an episode.
____
Conclusion
The hope for this episode is to make you wonder more about something you interact with daily. It isn’t about “woo woo” or “fun fun” stuff. It was to make you marvel at something you need to have every day, just as I did while researching it. It’s endlessly fascinating to ponder that even the top scientists in the world still cannot explain why this world-covering molecule acts the way it does. I am not sure why, but I find it so genuinely fulfilling to be intertwined with a bit of magic.
I hope a bit of that fascination was transferred to you as well.
Remember, “Thus ice floats because we are here to witness it floating.”
It’s 60% of you; it’s essential to your DNA, and its peculiarities are necessary for life on Earth. Yet, on some level, it’s magic and hasn’t given up its secrets yet.
Yet, there it is.
Alright, I gotta go.
Until next time, be well, everyone!
All this talking has made me thirsty. Now I just have to figure out how to quench that feeling…
Sources:
https://www.yalescientific.org/2015/01/unsolved-mysteries-the-mpemba-effect/https://www.astronomy.com/science/where-did-earths-water-come-fromhttps://www.sciencedaily.com/releases/2011/04/110426091122.htm
http://www.spring8.or.jp/en/news_publications/research_highlights/no_54/#:~:text=Individual%20H2O%20molecules,between%20H2O%20molecules.
https://manoa.hawaii.edu/exploringourfluidearth/chemical/properties-water/hydrogen-bonds-make-water-stickyhttps://www.chemistryworld.com/features/the-weirdness-of-water/4011260.articlehttps://www.newsweek.com/2018/05/25/water-most-mysterious-substance-earth-and-scientists-have-new-explanation-929091.htmlhttps://skepticalinquirer.org/2023/06/the-rise-and-fall-of-the-mpemba-effecthttps://www.theguardian.com/global/2015/may/11/water-weirdest-liquid-planet-scientists-h2o-ice-firefightershttps://www.newscientist.com/article/2170303-h2oh-10-mysteries-of-waterhttps://www.livescience.com/3724-mystery-water.htmlhttps://www.a-higher-view.com/sacred-geometry-tetrahedron
https://www.sciencedaily.com/releases/2011/04/110426091122.htmhttps://www.reptileknowledge.com/reptile-pedia/what-would-happen-if-ice-sankhttps://www.planetary.org/articles/how-did-earth-get-its-waterhttps://www.scientificamerican.com/article/how-did-water-get-on-earth